Mo Diagram Of Co Molecule
O2+ is paramagnetic or diamagnetic? Diagram molecular orbital mo carbon bond monoxide diatomic n2 nitrogen cn theory heteronuclear molecules ion electron bonding cyanide diatomics less Carbonyls compounds orbitals bond classnotes
Diatomic Species | MO theory | Chemogenesis
D6.5 mos for heteronuclear diatomic molecules – chemistry 109 fall 2021 Diagram molecular orbital carbon energy level monoxide using bond mo oxygen order determine questions solved Mo diagram and characteristics of co molecule
Solved: using the molecular orbital energy level diagram o...
Molecular orbital diagram oxide orbitals nitric cl2 diatomic mo energy level molecule delocalized theory principles molecules electrons electron bonding valenceDiatomic species O2 orbital molecular paramagnetic diamagnetic electron ti2 orbitals bonding hybridization configuration molecule grandinetti electrons unpaired 3p orbitale anti diagrammi socraticCarbon monoxide molecular orbital diagram.
Orbital molecular monoxide carbonyl complexes metallo organometallic legameMonoxide carbon molecular orbital diagram mo configuration socratic o2 treatment chemistry energy By writing molecular orbital configuration for no,co,o2 moleculesMo diatomic heteronuclear molecules orbital energy molecule.

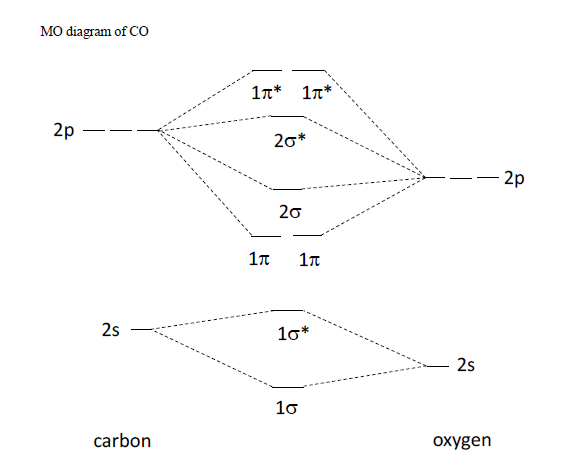
Mo diagram of co
Metal carbonyls9.8: second-row diatomic molecules Diagram mo energy level.
.


9.8: Second-Row Diatomic Molecules - Chemistry LibreTexts

D6.5 MOs for Heteronuclear Diatomic Molecules – Chemistry 109 Fall 2021

Diatomic Species | MO theory | Chemogenesis

Metal Carbonyls - Chemistry, Class 12, Coordination Compounds

MO diagram of CO - The Student Room
Solved: Using The Molecular Orbital Energy Level Diagram O... | Chegg.com

O2+ is paramagnetic or diamagnetic? | Socratic

MO Diagram and Characteristics of CO Molecule | Part-2 | by Ved Sir